by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Nov 7, 2018 | FDA enforcement
 The FDA’s recent press release announcing its activities in Operation Pangea XI, an enforcement operation to “crack down on websites selling illegal, potentially dangerous drugs; including opioids,” is conspicuously inaccurate about CanadaDrugs.com, which was forced to close its operations back in July. For a more general analysis of Operation Pangea XI, see my post from last week.
The FDA’s recent press release announcing its activities in Operation Pangea XI, an enforcement operation to “crack down on websites selling illegal, potentially dangerous drugs; including opioids,” is conspicuously inaccurate about CanadaDrugs.com, which was forced to close its operations back in July. For a more general analysis of Operation Pangea XI, see my post from last week.
CanadaDrugs.com vs. CanadaDrugs
CanadaDrugs.com was the biggest Canadian online pharmacy for well over a decade. During its 17 years in business, it estimated that people ordered one billion dollars in medicines. During that time, not a single adverse reaction was ever reported by the FDA or any health agency. Their business relied on the fact that Americans are never prosecuted for personal drug importation. The law permits, and Congress has recognized the importance of, the FDA using enforcement discretion to allow personal drug imports so people can afford medicine.
(more…)
Tagged with: canadadrugs, Enforcement, Operation Pangea
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Nov 1, 2018 | FDA enforcement
Last week, the FDA reported on its enforcement efforts against illegal and “potentially dangerous” online drug sales in Operation Pangea XI, a global initiative run by INTERPOL in cooperation with over one hundred drug regulators. Also, President Trump signed H.R. 6, The Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. I wish it was all about stopping rogue online pharmacies and ending the opioid crisis, but it’s not.
How Pangea XI and the SUPPORT Act are Related
The FDA is highlighting the SUPPORT Act and its effort in Pangea as important parts of the solution to stopping illegal online sales of addictive opioid drugs. The SUPPORT Act gives the FDA new authorities to stop illegal drug imports. So, what does this have to do with safe personal drug importation from pharmacies that require a prescription?
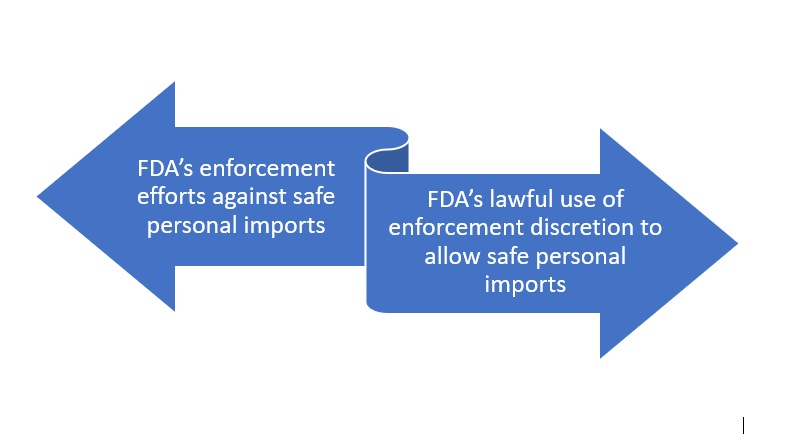
Before continuing, I can’t help note and show you that INTERPOL’s Pangea is funded by drug companies for this work. It’s this Pharma-funded initiative in which FDA plays a crucial role. The FDA does focus resources on shutting down some bad rouge sites, but it seems to also assist PhRMA in ways that curtail online access by Americans to lower-cost medicines from pharmacies in other countries.
There seems to be a tug of war: FDA’s enforcement efforts against safe personal imports vs. FDA’s lawful use of enforcement discretion to allow safe personal imports.
 (more…)
(more…)
Tagged with: Enforcement, Interpol, Operation Pangea, opioid
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Aug 31, 2018 | FDA enforcement
 The FDA is warning 21 online pharmacies to stop selling certain prescription opioid drugs to people in the U.S.
The FDA is warning 21 online pharmacies to stop selling certain prescription opioid drugs to people in the U.S.
According to the FDA, the 21 websites at issue are operated by four separate networks:
CoinRx,
PharmacyAffiliates.org,
PharmaMedics,
and MedInc.biz.
Each network received a similar warning letter from the FDA, which singles out their alleged illegal sales of unapproved and misbranded tramadol, also noting that a prescription was not required.
(more…)
Tagged with: Enforcement, fentanyl, opioids, tramadol, warning letters
 The FDA’s recent press release announcing its activities in Operation Pangea XI, an enforcement operation to “crack down on websites selling illegal, potentially dangerous drugs; including opioids,” is conspicuously inaccurate about CanadaDrugs.com, which was forced to close its operations back in July. For a more general analysis of Operation Pangea XI, see my post from last week.
The FDA’s recent press release announcing its activities in Operation Pangea XI, an enforcement operation to “crack down on websites selling illegal, potentially dangerous drugs; including opioids,” is conspicuously inaccurate about CanadaDrugs.com, which was forced to close its operations back in July. For a more general analysis of Operation Pangea XI, see my post from last week.


 The
The 