by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | May 1, 2020 | Drug Safety
In late March, drug giant Bayer Pharmaceuticals, out of the goodness of its heart, agreed to donate three million tablets of chloroquine phosphate to help the Trump administration get this drug into U.S. pharmacies. That’s according to an investigative article in Vanity Fair by Katherine Eban, called:
“‘Really Want to Flood NY and NJ’: Internal Documents Reveal Team Trump’s Chloroquine Master Plan”
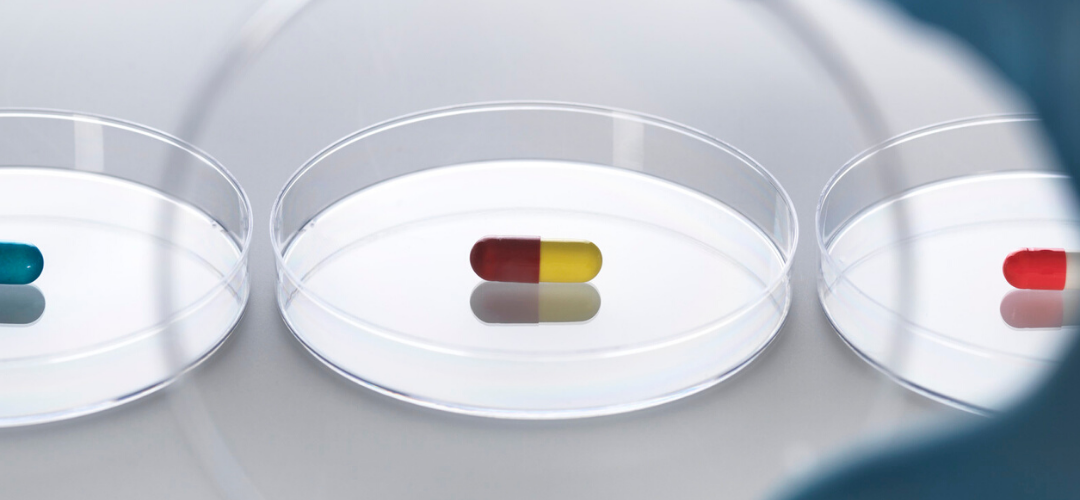
How inflammatory?! For Trump lovers, that title has the ring of just more biased, liberal media Trump-bashing. For Trump haters, it’s another reason to hate Trump. This post isn’t about our constant partisan divide or even Bayer or other evil drug companies, although they are referenced. It’s also not about unproven treatments for Covid-19 (that was last week). All parties should be interested in the story behind the story: independent drug testing and its potential importance to determine if a drug is safe and effective.
I’m a big fan of testing prescription drugs to see if they have the right stuff (to put it eloquently). Here’s why: lots of pharma-funded groups and some people at the FDA say don’t buy medicine online from foreign countries because it’s allegedly unsafe. Yet, independent testing of about a thousand prescription drug orders has shown that personal drug importation can and is done very safely. Periodic testing of foreign online pharmacy medication orders over the last decade has proven the efficacy of brand name Celebrex, Lipitor, Nexium, Viagra and Zoloft; generic ciprofloxacin and atorvastatin; and, just last month, hydroxychloroquine and chloroquine phosphate.
(more…)Tagged with: Bayer, Donald Trump, Katherine Eban, Roger Bate
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Jul 20, 2018 | Drug Importation

Azar is proposing a discussion about allowing imports of single-source drugs to lower costs
Yesterday, Health and Human Services Secretary Alex Azar announced that he was tasking FDA Commissioner Scott Gottlieb with forming a working group to explore how drug importation could be used to lower prices. See Gottlieb’s remarks on the proposal.
The crux of the proposal is very narrow. Azar is considering allowing imports of foreign versions of off-patent medicines that only one manufacturer (also referred to as “single-source” drugs) is selling in the U.S. market. That would be a drug without any competition where the company with the marketing license jacks the price. Keep in mind that he has simply called for a working group to discuss it.
I’m getting asked a lot of questions about this proposal and realize that many people, including well-informed journalists and policy professionals, don’t really get this.
People who already import medicines, through buying them online or carrying them home from Canada to save money may also be confused!
So, to help any and all understand what HHS and the FDA are considering when it comes to drug importation, below are some important takeaways. My general take, as noted in the Washington Post, is that it’s a step in the right direction (if it goes forward), and it could help educate the public about greater potential benefits to larger scale importation.
- This is not legalizing buying cheaper, FDA-approved meds from retail pharmacies in Canada online or otherwise.
- Millions of Americans already benefit from importing lower-cost, safe and effective medicines for personal use. They do this despite the existing federal prohibitions and scare tactics employed by industry-funded groups to deter such purchases. To do so safely, they stick to credentialed online pharmacies, such as those verified by PharmacyChecker.com. Today, Roger Bate, who is affiliated with the American Enterprise Institute, wrote: “All the FDA has to do is allow Pharmacy Checker to do its job and tell the American people about it.”
(more…)
Tagged with: Alex Azar, politics, Roger Bate, Scott Gottlieb
by Lucia Mueller, President, PharmacyChecker.com | Nov 30, 2017 | Drug Safety
 The verdict is in: blister packs may be a safer bet for packaging your medication. In the U.S. and sometimes in Canada, however, medications are dispensed as loose pills.
The verdict is in: blister packs may be a safer bet for packaging your medication. In the U.S. and sometimes in Canada, however, medications are dispensed as loose pills.
According to the FDA, over 1.3 million people are injured each year due to medication mistakes, which include dispensing errors in U.S. hospitals and pharmacies. As discussed in a blogpost by Roger Bate, an economist at the American Enterprise Institute, it may be safer to receive your medications in blister packs, whether dispensed locally or from an international pharmacy by mail, and the FDA seems to agree.
(more…)
Tagged with: Blister Packs, FDA, Roger Bate