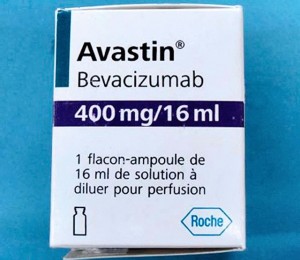
Counterfeit Avastin
According to the FDA, a counterfeit version of Avastin 400mg/16mL, a cancer treatment injectable medication, is being distributed in the United States, and “may have been purchased and used by some medical practices in the United States.” The fake drug “is labeled as Avastin, manufactured by Roche”, but it does not contain the medicine’s active ingredient, and is ineffective according to Roche. The counterfeit Avastin has “batch numbers that start with B6010, B6011 or B86017” and part of its label is written in French.
While it isn’t clear of how large the counterfeit supply is, the FDA has sent notice letters to 19 medical practices across the United States who may have administered the counterfeit drug to its patients, which could lead to adverse health effects. This counterfeit incident is different than one we reported on in 2010, when the FDA warned consumers about Generic Tamiful sold by an online pharmacy. In this case the Internet does not appear to play a role, rather the counterfeit drugs seem to have directly infiltrated the U.S. drug supply.
Price comparisons for Avastin are not available on PharmacyChecker.com, as its price listing participants do not offer this product for sale. Avastin is most often administered by a healthcare professional in a clinical setting not by the patient.
The reporting and disposal recommendations to healthcare professionals and patients by the FDA is as follows:
Medical practices that have obtained products from Volunteer Distribution and Quality Specialty Products should stop using them and contact the FDA. These products should be retained and securely stored. To report suspect counterfeit products and other suspect products obtained from Volunteer Distribution or QSP/Montana Health Care Solutions:
· Call FDA’s Office of Criminal Investigations (OCI) at 800-551-3989,
· Visit OCI’s Web site (www.accessdata.fda.gov/scripts/email/oc/oci/contact.cfm), or
· Email – DrugSupplyChainIntegrity@fda.hhs.gov
Healthcare professionals and patients are encouraged to report adverse events related to the use of suspect injectable cancer medications to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program:
· Complete and submit the report Online: www.fda.gov/MedWatch/report.htm
· Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178


