by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Nov 20, 2015 | Advocacy, Drug Importation, Drug Prices, FDA, Government

Today, as the Obama administration hosted a “public” forum (think invitation only) about pharmaceutical innovation, access and affordability, I announced the formation of a non-profit organization dedicated to helping Americans get justice when it comes to prescription drug prices: Prescription Justice Action Group (PJAG). Whereas the administration’s public forum ignored personal drug importation, PJAG is providing guidance to Americans on what to do if their prescription drug orders are refused import by the FDA so they can try to have their medications released.
For about fifteen years, tens of millions of Americans have purchased medication from outside the U.S. –usually ordering it online. They do it because they want to save money or they really cannot afford the medication here at local pharmacies. The fact is that it has become a lifeline of lower cost medications for Americans.
But a new law – Section 708 of the Food and Drug Administration Safety and Innovation Act – gives the FDA expanded powers to destroy your personally imported medications, whether bought from a Canadian, Indian, Turkish or U.K. pharmacy. That doesn’t mean they will. It just means that they can. That law became effective over a month ago, and we haven’t heard of increased FDA seizures and destructions of international prescription orders.
The FDA has stated, and we have re-affirmed on our blog and main website, that under most circumstances it’s technically illegal to import prescription medication for personal use. But is it really? Is it always?
Section 708 allows the FDA to detain and potentially destroy your prescription order if it appears to be misbranded, unapproved, counterfeit or adulterated. If they take your adulterated or counterfeit drugs then the FDA has done their job. Misbranded or unapproved drugs, in contrast, could be entirely safe and effective medications, the same or foreign versions of the ones you buy in the U.S., but much less expensive. Under Section 708, you must be notified by the FDA if they take your prescription drug import, and you have 20 days to challenge them on their action. PJAG, in consultation with legal advisers, believes that you can make a good case that FDA should not destroy the medication but instead send it to you.
There are many dangerous online pharmacies out there from which you don’t want to buy or import medication. We call them rogue online pharmacies. But if you import a genuine, safe and effective medication, one that was purchased from a PharmacyChecker.com-approved online pharmacy and you get a notification from the FDA telling you that your prescription drug order is subject to destruction…PJAG!
Tagged with: affordable prescriptions, Drug Importation, FDA, Food and Drug Administration Safety and Innovation Act, international pharmacies, Online Pharmacies, pjag, Section 708
by PharmacyChecker.com | Oct 9, 2015 | Drug Importation, Drug Prices, FDA
“Abstract pills” by Robson – Flickr
We issued a press release yesterday about our new drug price savings analysis, which shows that consumers can save 84% on average among a basket of 10 popular branded maintenance medications if purchased from verified international online pharmacies instead of local U.S. pharmacies. Many of the savings are over 90%! The greatest savings is 94% for the acid-blocking drug Nexium ($946.50 in the U.S. vs. $53.09 online for a three month supply of 40 mg pills) and the cholesterol-lowering drug Crestor ($803.89 vs. $51.40 – 20 mg pills). The greatest dollar savings is for the antipsychotic drug Abilify ($3,178.99 vs. $237.05 – 10 mg pills). The average annual savings per drug is $3,479. Despite this, last month, the U.S. FDA announced a “new rule” regarding its expanded authority to destroy personally imported medicine under Section 708 of the Food and Drug Administration Safety Act of 2012. Several members of congress have raised concern that FDA’s rules may impede access to affordable medication. [1]
The FDA says that its new regulation is meant to protect patients from unsafe medications and counterfeit drugs but the agency doesn’t seem to say how they will distinguish safe from potentially unsafe personal drug imports.
For the full press release, click here.
Prices for a 3-month supply of top-selling brand name medications
| Drug |
Local U.S. Pharmacy Price |
International Online Pharmacy Price* |
International Online Savings |
Annual Savings |
| Nexium 40mg |
$946.50 |
$53.09 |
94% |
$3,573.64 |
| Crestor 20mg |
$803.89 |
$51.40 |
94% |
$3,009.96 |
| Abilify 10mg |
$3,178.99 |
$237.05 |
93% |
$11,767.75 |
| Advair Diskus 250/50mcg (180 doses) |
$1,203.00 |
$99.99 |
92% |
$4,412.04 |
| Spiriva Handihaler 18mcg |
$1,221.00 |
$113.99 |
91% |
$4,428.04 |
| Diovan 80mg |
$611.99 |
$57.85 |
91% |
$2,216.56 |
| Synthroid 100mcg |
$137.99 |
$26.99 |
80% |
$444.00 |
| Jardiance 10mg |
$1,150.00 |
$287.99 |
75% |
$3,448.04 |
| Ventolin HFA 100mcg |
$192.00 |
$68.82 |
64% |
$492.72 |
| Lantus Solostar 15ml |
$397.89 |
$148.94 |
63% |
$995.80 |
| Average |
$984.33 |
$114.62 |
84% |
$3,478.85 |
Sources: Local pharmacy prices based on prices at chain drugstores in New York City; International online pharmacy prices based on lowest prices listed on PharmacyChecker.com. All prices obtained on September 30, 2015.
*Medications dispensed by licensed pharmacies, verified by PharmacyChecker.com, in one of the following countries Australia, Barbados, Canada, India, Mauritius, New Zealand, Turkey, Singapore, or United Kingdom.
[1] U.S. Senator David Vitter, “Vitter Fights to Keep Prescription Drug Prices Affordable Through Reimportation,” July 9, 2014 [press release], see [www] vitter.senate.gov/newsroom/press/vitter-fights-to-keep-prescription-drug-prices-affordablethrough-reimportation [Last accessed 9/20/14]. 38 Representative JoAnn Emerson (MO), “Food and Drug Administration Reform Act.” May 30th 2012. See [www] votesmart.org/public-statement/702416/food-and-drug-administration-reform-act-of-2012#.UxVJN-co4s9 [Last accessed 9/22/14]. Letter to the U.S. Food and Drug Administration by Congressman Keith Ellison dated July 1st, 2014. See See [www] regulations.gov/#!documentDetail;D=FDA-2014-N-0504-0022.
Tagged with: brand name drugs, Drug Prices, FDA, Food and Drug Administration Safety Act, international pharmacies, Online Pharmacies, personal drug importation
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Aug 12, 2015 | Advocacy, Cancer Drugs, Counterfeit Drugs, Drug Importation, FDA, Government, Personal Drug Importation
Almost three years ago, we blogged about a federal investigation of CanadaDrugs.com, which for many years has safely sold prescription medication at prices far lower than typically available in the U.S, and which is a verified online pharmacy in the PharmacyChecker.com Verification Program. The investigation focused on CanadaDrugs.com’s wholesale drug importation and distribution to doctors and clinics — an area CanadaDrugs.com has long since exited. It did not focus on CanadaDrugs.com’s retail sales to consumers for personal use, which is the focus of the PharmacyChecker.com Verification Program and the information we provide to consumers on our website about online pharmacies.
Recently, an indictment was unsealed in federal district court in Montana that charged CanadaDrugs.com, Ltd. (the entity which owns CanadaDrugs.com) and others with illegal wholesale drug importation, which allegedly occurred between three to six years ago. The allegations include wholesale distribution of a counterfeit version of the cancer drug Avastin to medical clinics in the U.S.
The indictment of CanadaDrugs, Ltd, comes as no surprise, as the investigation was well publicized. It will also come as no surprise, however, when the U.S. pharmaceutical industry tries to use the charges, which focus exclusively on wholesale drug importation, in an effort to discredit safe personal drug importation. As we have written here and opined in the New York Times, the U.S. pharmaceutical industry and pharmacy chains feel threatened because Americans can and do safely purchase their medications online at substantially lower cost from pharmacies in other countries. Thus, the industry, the “non-profit” groups it funds, and the government agencies which it lobbies and seeks to influence, will see this indictment as yet one more opportunity to scare people from personal drug importation. This slight of hand is wrong, since the investigation and indictment have nothing to do with personal drug importation. In fact, even the Wall Street Journal, which was instrumental in publicizing the investigation, clarified the difference between wholesale businesses and CanadaDrugs.com: “There is no indication that fake medicines were sold through the company’s consumer-focused website, CanadaDrugs.com.”
(more…)
Tagged with: avastin, canadadrugs, kamath, montana
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Aug 10, 2015 | FDA, Medication non-adherence, Online Pharmacies, Personal Drug Importation
Last week, Bing announced a new effort to use its search engine to warn consumers about threats from “fake” online pharmacies. The big problem is that at least some of the online pharmacies they list are not fake, but represent very real, licensed pharmacies, ones that require valid prescriptions and have been safely helping Americans afford medication for years. We know this because these pharmacies have been carefully evaluated, inspected, and monitored by us at PharmacyChecker.com, and meet high standards of pharmacy practice. See our standards: http://www.pharmacychecker.com/verification_program_guide_and_standards_1_3.pdf.
The online pharmacies targeted by Bing, which are approved in our program, are all located outside the U.S. Curtailing online access to lower cost and safe foreign medication using scare tactics is a strategy employed by the pharmaceutical industry. Indeed, Bing’s action seems to have a lot to do with stopping safe personal drug importation and will recklessly alarm Americans so they don’t buy more affordable medication from other countries.
Here is the warning which appears when a consumer’s Bing search results include links to one of these online pharmacies and they try to click on the link to that online pharmacy:
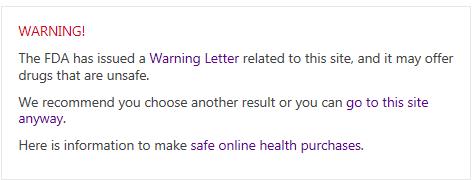
To choose the pharmacies it targets, Bing is relying on a list of online pharmacies which have received warning letters from the FDA. But, in at least several cases, these warning letters, which you can find on the FDA’s website, do not indicate a pharmacy to be fake, nor do they pertain to sales of counterfeit or adulterated medications, nor to any problems with the pharmacy meeting good standards of practice. Instead they relate to 1) sales of lawfully manufactured generic versions of drugs that are still on patent in the U.S., and 2) medications that are approved in Canada but not in the U.S. We will examine each of these letters fully over the next week, but our initial review indicates that these issues have been addressed by the online pharmacies in our Verification Program that received the letters.
The genesis of Bing’s action, which is most likely coordinated with the FDA, comes from the scare tactics about foreign medications conceived by pharmaceutical companies and their lobbying largesse. Why else would Bing decide to target only online pharmacies when many other pharmacies, pharmaceutical companies, medical device manufactures, and dietary supplement distributors have also received FDA warning letters for various infractions, yet Bing does not target them with its pop-up warning?
Bing’s actions would be great if the websites it is targeting were all fake or rogue online pharmacies, but they are not. When consumers see Bing’s warning, they will likely do one of three things:
- Keep searching for another online pharmacy that charges a price they can afford. They may find one of the tens of thousands of rogue pharmacy websites that don’t require a prescription (but are not included on FDA’s new list) and buy from that one. Then they are far more likely to end up with a counterfeit drug.
- Go to their local big chain pharmacy and pay hundreds if not thousands of dollars more for their prescribed medication. The Warning has a link to “safe online health purchases” but those take you to U.S. online pharmacies only, which are often the websites of the big chain pharmacies!
- Not take their prescription medication at all. Thirty-five million Americans each year already forgo prescribed medication due to cost.
These are horrible outcomes. Yes, warning Americans about rogue online pharmacies is good public policy. But leading Americans away from safe personal drug importation will just lead to fewer people getting medications they need, more Americans choosing between food and medicine, and larger profits for the big drug companies.
For those interested in a full policy analysis of FDA’s current campaign, please see our report called: “Online Pharmacies, Personal Drug Importation and Public Health.”
Tagged with: Big Pharma, Bing, FDA, international online pharmacies, PharmacyChecker Verification Program, rogue online pharmacies, search engines
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Jul 8, 2015 | Controlled Drugs, FDA
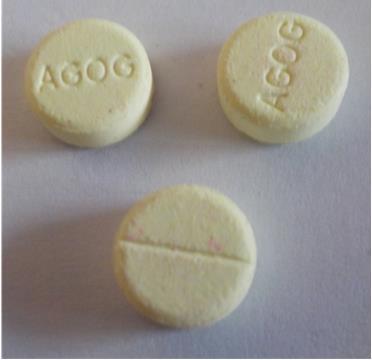
This diazepam – generic Valium – is really haloperidol.
The U.S. Food and Drug Administration (FDA) issued a warning last week that a drug product sold in Central Africa, mislabeled as diazepam, which was actually the drug haloperidol (for schizophrenia), had caused 700 adverse reactions, such as acute contractions of the muscles in the face and neck. While there are no reports that the product entered the U.S., FDA cautioned Americans who take diazepam that it could, potentially, be sold over the Internet and to be on the lookout for the pills you see in the image to your left. Sound advice!
For the backstory checkout the World Health Organization’s Medical Product Alert.
Diazepam is the generic name for the anti-anxiety medication commonly known as Valium. It is a controlled prescription drug, meaning one associated with abuse use and addiction.
We recommend to U.S. and all consumers that you not buy controlled medication internationally from an online pharmacy. Online pharmacies that sell Valium, and all controlled drugs, internationally are not eligible for the PharmacyChecker.com Verification Program. (more…)
Tagged with: diazepam, FDA warning
by PharmacyChecker.com | May 29, 2015 | FDA, Government, Internet Censorship, Online Pharmacies, Policy, Public Health
Tens of millions of Americans cannot afford medication, which can lead to more sickness, hospitalizations, and even death. Despite this public health crisis, our trusted regulatory authorities, the pharmaceutical industry, and U.S. pharmacy trade groups work together to scare Americans away from ordering much more affordable medications from foreign pharmacies. Is that right or wrong?
This week, in our continuing quest to get the truth out and for our elected leaders in Congress to take bold action to protect online access to safe and affordable medication, we’re publishing the next section of our report called Online Pharmacies, Personal Drug Importation, and Public Health…
(more…)
Tagged with: BeSafeRx, Canadian Internet pharmacies, FDA, GAO, ICANN, NBAP, safe online pharmacies






