 I write this post about insulin with ambivalence and frustration, but also hope. The diabetes patient and activist community is rightfully seething, screaming at the top of their lungs about high insulin costs in America. One young man stands out in my mind. He recently died because he could not afford his insulin, and now his mother bravely speaks out about her son’s death—an example of why drug prices are a national crisis.
I write this post about insulin with ambivalence and frustration, but also hope. The diabetes patient and activist community is rightfully seething, screaming at the top of their lungs about high insulin costs in America. One young man stands out in my mind. He recently died because he could not afford his insulin, and now his mother bravely speaks out about her son’s death—an example of why drug prices are a national crisis.
In a March article in Insulin Nation, the journalist/editor Audrey Farley and I discussed the issue of buying more affordable insulin online from pharmacies located in Canada and the U.K. She found that many of her readers were doing so and wanted to let them know how to go about it safely.
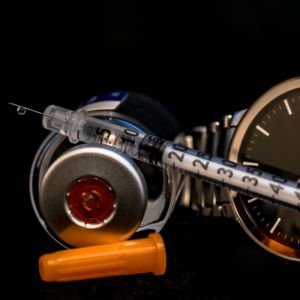
One carton of Lantus Solostar (5 pens of 3ml each), a long-acting insulin made by Sanofi Aventis in Germany, goes for about $430 at a CVS in Brooklyn, New York. At Rexall Drugs in Toronto, the price is $84.99. That’s 80% less. Until recently, a small number of Canadian pharmacies in our Verification Program sold it for about $180. The online Canadian price is higher because of the fees associated with special packaging and shipping – but it’s still 56% less than the U.S. price. For those prices, Americans living with diabetes could save a couple of thousand dollars a year; and those who can’t afford it here at all could stay alive.
Insulin is a temperature-sensitive medication and, because of that, requires significant precautions when shipping. To prevent it from degrading requires special packaging. Effective patient communication is also necessary. Because of our recent updates to our refrigerated medications policy, one that places stricter requirements on pharmacies in the PharmacyChecker Verification Program, most will likely choose not to sell insulin to Americans— at least not for a while. Here’s why…
Our former policy required the attention and care in line with, and even beyond many, U.S. state pharmacy standards. It banned (and continues to ban) pharmacies outside Canada, the U.K. and the U.S. from shipping insulin and other medications requiring refrigeration in the U.S. For those pharmacies in Canada, the U.K. and the U.S., the policy required cold packs in the shipment, a temperature indicator for the patient to review, and patient communications on how to read the indicator to see if the product is still good.
Our current policy takes it further. It requires pharmacies to ensure that their insulin shipments will not deviate from 2 to 8 degrees Celsius. Pharmacies must use optimal packaging for cold chain dispensing and temperature indicators that show the patient if the product froze during shipment or was exposed to heat in a manner that may compromise the product, in accordance with the specifications on the manufacturer’s label. The shipping method is verified by PharmacyChecker by requiring a demo shipment (without the medication) from the pharmacy. Our new standards also require a pharmacy shipping insulin to include the following patient notification on web pages where insulin is sold:
“This product requires special packaging to maintain its integrity during the shipping process. DO NOT USE THIS MEDICATION if the attached temperature indicator shows that the medication was exposed to temperatures below 2 degrees or above 8 degrees Celsius, and contact the pharmacy immediately.”
Dr. Shivam Patel, our director of pharmacy verification and information, is a doctor of pharmacy and licensed pharmacist in Massachusetts. He has reviewed the strongest U.S. state pharmacy refrigerated medication standards and laws and found none are as strict as the refrigerated medications policy imposed by PharmacyChecker.
For the past few years, very few pharmacies in our program have elected to ship insulin to the U.S., in part because of the strictness of our policy but also because they are afraid of the optics of a bad shipment of insulin. The FDA could intercept an order and potentially find that the insulin is compromised, then use this occurrence for publicity against all personal importation of medication.
Sadly, U.S. pharmacies don’t always take care in shipping and even properly storing insulin. However, when they make a mistake, they are probably not going to receive a letter from the FDA asking them to stop dispensing insulin, or any medications at all, to U.S. patients.
We don’t want to ban Canadian pharmacies that are lawfully permitted and able to dispense by mail more affordable insulin to patients in the U.S. who cannot afford it here. However, for the reasons stated above, we see the the necessity in making our pharmacy standards for international shipping of insulin higher than any here in the U.S. for domestic shipping of insulin.
Advocacy Call to Action
Our policy can be met. One problem for Canadian pharmacies and U.S. patients is that Federal Express and UPS may not overnight ship insulin from a pharmacy in Canada to a patient in the U.S. It is my belief that the couriers are warned by the FDA not to take such shipments due to technical prohibitions against personal imports.
I suggest that the diabetes community use its collective voice to ask that those couriers for clarification on their policies related to shipping insulin. Guaranteed overnight shipments would make it easier for licensed pharmacies in Canada to meet our policy and it would be just as safe, if not safer, than ordering from U.S. mail order pharmacies.
Furthermore, according to the website of Federal Express, “Importation of prescription drugs by an individual U.S. consumer for personal use is prohibited unless FDA approved.” Is Lantus Solostar an FDA approved drug? The answer is yes, but the label in Canada is different therefore the FDA deems it “misbranded” if sold in the U.S. However, a U.S. patient with the U.S. label in hand, and a prescription from their provider, should be able to overcome the designation of “misbranded” and legally import the drug. Food for thought.
Finally, I call on the community of diabetes patients and activists to weigh in on our policies and recommend modifications. If you and your providers can get behind a sufficiently strict standard for Canadian pharmacies to ship insulin to Americans who cannot afford it here, then please let us know.
Let’s talk!
Tagged with: #insulin4all, FedEx, Insulin, PharmacyChecker Verification Program


My wife’s diabetes Dr. will no longer give her the prescription for her insulin.He says he has to send it directly to the pharmacy where she buys her other medications. She won’t be able to buy insulin from Canada now (that is after the warm months).
Just an FYI… I’m not sure about shipping it but when I’ve crossed the border to purchase insulin, the pharmacy does not require any prescription. I just need to call 24 hours in advance to make sure they have the necessary quantities on hand. For us, the cost savings is totally worth the 2 hour drive each way.
Hi Loren and Martin, It’s true. Read our other post regarding driving to Canada to buy insulin: https://pharmacycheckerblog.com/buying-insulin-canada-without-prescription
Am I able to bring my Insulin back to the US in my “vehicle” without any border issues or declaring the items and duty cost? 724-202-6399
When you’re crossing the border, the U.S. Customs Border Patrol (CBP) is not allowed to stop the importation of FDA-approved medication from Canada for personal use – even though it’s technically prohibited? See: Public Law 115-31. Read more here: https://pharmacycheckerblog.com/buying-insulin-canada-without-prescription
Loren– Where do you live? Even in New York, where e-prescribing laws have become quite strict, if you intend to have your prescription filled out-of-state (Canada included), a doctor can legally hand you a paper prescription.
This is garbage. Unless Medicare is paying for it (and obviously, this is not happening), there is zero requirement that a prescription must be sent directly to the pharmacy. Tell her to call the doctor’s office and demand a prescription or seek a new doctor. Nobody should be held hostage by their doctors by limiting their choice of where to purchase drugs.
You don’t need a prescription to buy it in Canada. This is what I read.
@Loren. Do you know who established this policy? The provider? The clinic?
Can I purchase insulin in Canada
Do I need a prescription
Can you send it or do I need to pick it up
Hi Joyce- PharmacyChecker.com is not a pharmacy. We verify international online pharmacies for your safety when shopping for meds online. You must have a prescription to buy insulin through a PharmacyChecker-verified Canadian online pharmacy. Many of our pharmacies have chosen not to sell insulin given PharmacyChecker.com’s strict policies on shipping temperature-sensitive medications. You may be interested in our blog post regarding buying insulin in Canada: https://pharmacycheckerblog.com/buying-insulin-canada-without-prescription
There is pharmacy located in Vancouver, BC Canada that ships insulin to the U.S that take about three days and is ship in special packaging and will not ships during summer months when weather is hot, do you know if this pharmacy is rated. Marks Marine Pharmacy is the name of the pharmacy
Hi Tommy, Marks Marine Pharmacy is not currently verified and monitored by PharmacyChecker.com. We recommend you stick to only those pharmacies that are verified, meeting critical safety standards to protect patient health when buying medication online: https://www.pharmacychecker.com/online-pharmacy-ratings/
Marks Marine is the one I had been told about too. Did you order from them anyway? How did it arrive?
I order 6 box’s before warm weather and it was in my hands in 3 days and way cheaper
Did you have to have a prescription? I can’t afford the 155 dollars for a doctors appointment. I getting scared my sugar is out of control over 400 for a couple days now. Ugh
Marks isn’t shipping insulin to the USA anymore. I called and spoke to the pharmacist.
Marks is infact shipping Insulin to America. I have ordered many times, for several years, and have never had an issue. Insulin arrives cold and within 3-4 days!
I thought Marks is still shipping though they dont guarantee the product quality upon shipping process.
I bring insulin to my siblings in LA let me know if you need a hand bringing it down.
will they.. mark’s .. ship insulin to St. Louis in 3 days?
Mark’s Marine is not currently accredited and monitored through the PharmacyChecker Verification Program, therefore we are not able to assess if it satisfies high standards of online pharmacy practice or the amount of time it takes them to ship insulin to St.Louis.
hello everyone,
I reside in BC, Canada and is looking to provide product transport. I do that for my sibling in LA. Thought the drive is quite far its worth it.
I bought a temperature controlled fridge and so I feel much safer in handling it myself since Mark’s doesnt guarantee the end condition of it once upon arrival.
If anyone needs a hand let me know.
hi i live in fremont ca.could you drop some off to me on your trip to la?
Should I declare that we bought a 90 day supply of insulin for personal use when going through US Customs from Canada?
Hi Gary –
As I understand it, Customs and Border Protection (CBP) does not stop people from personally importing small quantities of prescription drugs on their person from Canada, including if you declare the import. In fact, under current law, CBP cannot use federal funds to stop you from importing FDA-approved drugs from Canada for your own use, up to a 90 day supply. Certain drugs, such as biologics and controlled drugs are not protected. I recommend that you read up on how prevalent a practice it is and not something that people get into trouble for: https://www.washingtonpost.com/world/the_americas/as-price-of-insulin-soars-americans-caravan-to-canada-for-lifesaving-medicine/2019/06/14/0a272fb6-8217-11e9-9a67-a687ca99fb3d_story.html.
PLEASE BEWARE of CanadianInsulin .com! From 2018 to 2021, I was a loyal customer. The situation that Canadian Insulin just put me through is shocking and heartbreaking. I placed an order for my diabetic kitty on Oct. 5th, and their negligent handling of my order, even after I had pleaded with them to overnight it after their mistake, arrived on Nov. 9th. They also shipped it out the same way they shipped out the first order and did not rush it to me. My cat needed to be hospitalized on Nov. 10th and she died on Nov. 15, 2021. They had ignored my calls and emails many times when I was trying to make sure they were processing my order. Karen H. and Jennifer S. apologized that my “recent order was shipped to the incorrect address” and will refund my $176 insulin order because I “did not receive my order to the correct address” and “no longer require this medication.” I told them I DO NOT want a refund on the insulin and showed them my $2,540.79 vet bill to which they refused because “these circumstances are outside of our control.” I repeatedly said no to the insulin refund, and they processed it anyway.
While my first order went to the wrong address on Oct. 26, they did not offer me a refund then. It took my cat dying on Nov. 15 for them to force a refund saying it’s for the shipping issue when in reality it’s because they know they are the reason she died.