by PharmacyChecker.com | Mar 30, 2012 | Drug Importation, Drug Prices, Online Pharmacies, Online Pharmacy Verification Services, Saving Money on Prescription Drugs
A new study published by the National Bureau of Economic Research finds that pharmacies approved in the PharmacyChecker.com Verification Program sell safe medication and can help Americans achieve substantial savings. By filling 370 prescriptions through 41 online pharmacies and testing the authenticity of the medications received, independent researchers from the American Enterprise Institute in Washington, D.C. found no test failures among online pharmacies verified by third-party verifiers, such as PharmacyChecker.com.
Since all online pharmacies approved by PharmacyChecker.com – whether American or foreign – are licensed, require a prescription, and meet high standards of online pharmacy safety, the results of this study are not the least bit surprising. Past studies have shown the exact same results. Still, the FDA ignores the facts and continues a consumer education policy recommending that Americans completely avoid non-US online pharmacies. We believe the FDA’s policy is unethical and unfair. More importantly, its policy is bad for the public health because less access to affordable prescription drugs means fewer Americans will procure needed medications.
For more on this study see our news release.
Tagged with: affordable prescriptions, Drug Prices, international pharmacies, Online Pharmacies, pharmacychecker.com
by PharmacyChecker.com | Feb 15, 2012 | Drug Prices
Counterfeit Avastin
According to the FDA, a counterfeit version of Avastin 400mg/16mL, a cancer treatment injectable medication, is being distributed in the United States, and “may have been purchased and used by some medical practices in the United States.” The fake drug “is labeled as Avastin, manufactured by Roche”, but it does not contain the medicine’s active ingredient, and is ineffective according to Roche. The counterfeit Avastin has “batch numbers that start with B6010, B6011 or B86017” and part of its label is written in French.
While it isn’t clear of how large the counterfeit supply is, the FDA has sent notice letters to 19 medical practices across the United States who may have administered the counterfeit drug to its patients, which could lead to adverse health effects. This counterfeit incident is different than one we reported on in 2010, when the FDA warned consumers about Generic Tamiful sold by an online pharmacy. In this case the Internet does not appear to play a role, rather the counterfeit drugs seem to have directly infiltrated the U.S. drug supply.
Price comparisons for Avastin are not available on PharmacyChecker.com, as its price listing participants do not offer this product for sale. Avastin is most often administered by a healthcare professional in a clinical setting not by the patient.
The reporting and disposal recommendations to healthcare professionals and patients by the FDA is as follows:
Medical practices that have obtained products from Volunteer Distribution and Quality Specialty Products should stop using them and contact the FDA. These products should be retained and securely stored. To report suspect counterfeit products and other suspect products obtained from Volunteer Distribution or QSP/Montana Health Care Solutions:
· Call FDA’s Office of Criminal Investigations (OCI) at 800-551-3989,
· Visit OCI’s Web site (www.accessdata.fda.gov/scripts/email/oc/oci/contact.cfm), or
· Email – DrugSupplyChainIntegrity@fda.hhs.gov
Healthcare professionals and patients are encouraged to report adverse events related to the use of suspect injectable cancer medications to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program:
· Complete and submit the report Online: www.fda.gov/MedWatch/report.htm
· Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
by PharmacyChecker.com | Feb 10, 2012 | Saving Money on Prescription Drugs
A new and portable strategy for saving money on prescription drugs has just hit the market with the LowestMed app from LowestMed.com. Our research finds that the most substantial savings consumers can expect are for generic medications, but far less so when it comes to expensive brand named drugs.
To help American consumers “Find the lowest price… fast” at a local chain pharmacy, LowestMed has created a free smartphone application that uses the current location of your phone to find the nearest pharmacy with the lowest price on a prescription drug that you need. According to the Washington Post, “LowestMed also comes with a free discount card, which can further reduce the price of a medication by between 10 and 85 percent.”
Testing out this new savings strategy, we find that a 30-day supply of Lisinopril 10mg can cost anywhere from $10.00 (Target) to $36.63 (CVS) – the app not only helps you find the $26.63 savings – 73% but also to map the location for you.
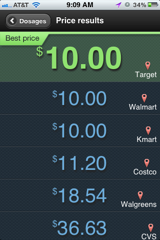
While savings like those on Lisinopril are great, many consumers may need to turn to verified international pharmacies when shopping for big brand names. Thirty tablets of Plavix 75mg, for example, cost $197.64 at the cheapest bricks and mortar pharmacy on LowestMeds.com, $205.10 at the most expensive. Saving $7.46 per month is nice, but saving $153.10 is not only much better but necessary for some Americans who could simply cannot afford the U.S. price! Plavix costs just $52 for a month supply at the lowest priced international pharmacy in the PharmacyChecker.com Verification program – a savings of 74%!
Tagged with: app, brand-name, bricks and mortar, CVS, discount card, free discount card, generic, international p, Lisinopril, LowestMed.org, Plavix, smartphone, Target, United States, Washington Post




