by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Jul 10, 2014 | Drug Importation, Online Pharmacies, Politics
In our recent public comments to the U.S. Food and Drug administration, we invoked your concerns with new FDA regulations to implement Section 708 of the Food and Drug Administration Safety and Innovation Act (FDASIA) for destroying safe (and affordable) medications imported for personal use. We issued a press release on the issue as well. Here are three of almost 2000 comments:
Morton Ross, Palm Harbor, FL 2014-04-03, “The Meds I take daily, are the difference between ‘Life and Death’. I cannot afford the higher prices at local pharmacies.”
Darilyn Schlie, Fort Worth, TX 2014-04-03, “Without the ability to go outside the US, I will not be able to afford the medication I need.”
James Marshall, Nashville, TN 2014-04-03, “I have emphysema and could not afford my medications if not for being able to order some of them from outside the USA.”
We need more voices! Working with RxRights.org, you can send a message to the Secretary of Health and Human Services stating your concerns, asking that she prevent morally unjust and dangerous refusals and destructions of imported drugs for personal use. The messaging process is easy and together we can win this!
The situation is not dire…yet. The regulation has not taken effect and it’s uncertain what its impact will actually be. Currently, the conventional wisdom holds that the chances of your prescription order being detained by U.S. Customs is less than 1%. What we don’t know is if the new regulations will change that dramatically.
What we do know is that FDA issued a proposed rule in May to implement Section 708, which is deceptively called “Destruction of Adulterated, Misbranded, or Counterfeit Drugs Offered for Import.” Destroying adulterated and counterfeit drugs sounds like a good plan but many, if not most, ‘misbranded drugs’ sold in foreign pharmacies are actually the same drugs you can buy at your local pharmacy just with different labeling and packaging. Though sometimes manufactured in a plant not registered with the FDA, they are manufactured in plants registered with another drug regulatory authority. Or they might be a generic version of a brand name drug that is approved in the U.S. but not yet off-patent here. These examples of real, safe and effective medications are usually deemed ‘misbranded’ or ‘unapproved’ by FDA. For more on this see my New York Times op-ed.
To Congress’ credit, before Section 708 goes into effect, regulations must be drafted requiring that consumers, 1) receive notification that their prescription drug orders have been refused import and 2) are provided “appropriate due process” to defend their drug imports before they are destroyed. Unfortunately, as I read it, FDA’s proposed rule did not assure consumers “appropriate” due process. In our comments we proposed that FDA clearly explain to consumers why their drug imports were refused and exactly how consumers can provide testimony to prevent the FDA from destroying their imported drugs. In the final rule, consumers should be able to successfully defend refused drug imports of safe and prescribed medication to have them released not destroyed. They need those medications to safeguard their health, and sometimes even lives.
We also proposed a revision to FDA’s personal drug importation policy so that safe personally imported medication from certain countries with very strong pharmaceutical regulations and pharmacy standards would not be detained or refused. We also recommended continuing actions to shutdown dangerous rouge pharmacy sites but not obtusely conflating them with safe international online pharmacies.
See below to learn more and advocate.
Petition the Government!
Comments by Americans concerned with Section 708
Comments by PharmacyChecker.com to the FDA on Section 708
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Jul 2, 2014 | Advocacy, Personal Drug Importation, Politics
 As we approach July 4th, a day to celebrate freedom in America, I urge you to stand up for your freedom to access safe and affordable medication!! Let’s face it: the global drug companies – big Pharma – would rather you pay higher prices for their medications because it makes them more money. In its infinite pandering to big Pharma, Congress included language in the Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA) – an otherwise pretty useful drug safety bill – expanding the authority of the U.S. Food and Drug Administration to destroy safe, personally imported medications. In the spirit of independence – life, liberty, and the pursuit of happiness – take this time to send a message to the Secretary of the Department of Health and Human Services (HHS) asking that she take the necessary actions to protect your prescription drug orders, ones ordered from safe international online pharmacies.
As we approach July 4th, a day to celebrate freedom in America, I urge you to stand up for your freedom to access safe and affordable medication!! Let’s face it: the global drug companies – big Pharma – would rather you pay higher prices for their medications because it makes them more money. In its infinite pandering to big Pharma, Congress included language in the Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA) – an otherwise pretty useful drug safety bill – expanding the authority of the U.S. Food and Drug Administration to destroy safe, personally imported medications. In the spirit of independence – life, liberty, and the pursuit of happiness – take this time to send a message to the Secretary of the Department of Health and Human Services (HHS) asking that she take the necessary actions to protect your prescription drug orders, ones ordered from safe international online pharmacies.
Thanks to RxRights.org for leading the charge on this effort!
The onerous language under discussion is found in Section 708 of FDASIA, which allows the FDA to destroy medication orders valued at $2500 or less that are refused import. The medications subject to refusal and destruction are those deemed “adulterated, misbranded or counterfeit.” Those words seem pretty scary but don’t be fooled. Unlike an adulterated or counterfeit drug, an imported ‘misbranded’ drug can be the same, safe and effective medication sold in a U.S. pharmacy but with a slightly different label. Seizing and destroying a person’s safe prescription drug order is immoral, anti-American, and dangerous to that person’s health.
There’s a catch in the law, which actually invokes the Spirit of 1776. Before Section 708 goes into effect, the HHS Secretary shall draft proposed regulations to provide consumers with due process to “challenge the decision to destroy the drug.” That means Americans should have an opportunity when their medication orders are seized to tell the government “don’t destroy my safe prescription drug order.” As the agency under HHS tasked with regulating the nation’s drug supply, it’s the FDA that leads the government in this process. FDA’s proposed regulations, which are open for public comment, were drafted and published in early May. While they fail to provide what the law requires – “appropriate due process” – I believe they leave the door open to amend what they have proposed. This weekend I’ll be working to submit PharmacyChecker.com’s public comments to try and assist (persuade?) the FDA to issue a more consumer-friendly final regulation that protects your access to safe and affordable imported medication.
I invoke the spirits of our Founding Fathers to guide us in this fight for independence from the tyranny of high drug prices.
Happy Fourth of July!
Tagged with: Due Process, Regulations, RxRights, Section 708
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Jun 25, 2014 | Drug Prices, Drug Safety, Generic drugs

Toprol XL, a prescription drug that treats high blood pressure and is shown to lower the risk of heart attacks, is in the news due to recalls of some generic versions and because some doctors are finding the generics doesn’t always work as well as the brand. Consumers taking a generic version (metoprolol succinate extended-release), might want to switch back to the brand, but that could raise their drug bills substantially
The issue has also re-ignited the topic of problems with Indian drug quality. Wockhardt and Dr. Reddy’s Laboratories, both based in India, have pulled 100,000 bottles of their respective metoprolol succinate extended-release products because their pills were not dissolving properly. For extended release drugs, this can be a big problem with a drug’s efficacy.
So where can you buy an American made version of this drug? I believe that would be very hard to do. Brand name Toprol XL sold on U.S. pharmacy shelves is a product of Swedish company AstraZeneca and, according to the drug’s labeling, made in India. There are American companies that make metoprolol succinate as well, such as Mylan, but they manufacture the drug in India, too. Some Toprol XL generics were made in America, but that didn’t work out so well due to manufacturing problems here at home.
Dollars and Sense – You can save 70% on the brand!
In the U.S., generic metoprolol succinate costs about 45 cents a pill. If you want brand name Toprol XL (100mg), the cost is about $2 apill. However, if you only want the brand name product and want to spend a lot less, it can be purchased from a verified international online pharmacy for as little as 60 cents a pill, just a bit more than the cost of a generic in the U.S., and a 70% savings on the U.S. brand price.
Compare Toprol XL drug prices on all strengths on PharmacyChecker.com.
Tagged with: AstraZeneca, Dr. Reddy's Labratories, Toprol XL, Wockhardt
by Gabriel Levitt, Vice President, PharmacyChecker.com and Sam Werbalowsky, Pharmacychecker.com | Jun 20, 2014 | Generic drugs, Pharmaceutical Industry
Americans are used to finding low-cost generic medications about six months to a year after they’re approved by the FDA. Think Lipitor, Plavix and Lexapro, which are all priced about 80% lower as generics.
Unfortunately, patients who had been looking forward to newly-approved generic versions of Nexium, Diovan, or Valcyte will have to keep waiting as a knot of legal and regulatory guidelines delay their U.S. release. The FDA approved Indian drugmaker Ranbaxy’s generic versions of these meds but has banned the plants to be used for their production from exporting products to the U.S., due to findings of substandard manufacturing practices.
You’d think that another drug manufacturer could just make and market the drug, but the U.S. approval system doesn’t allow that. The FDA grants six months of exclusive marketing rights to the company that first gains approval for a generic drug. We wrote about this process two weeks ago in our blog post covering generic Celebrex. Even though Ranbaxy’s plants can’t export generic versions of Nexium, Diovan, and Valcyte to the U.S., it still retains marketing exclusivity in the U.S. market!
This is good news for brand name drug makers and bad news for consumers. Global sales for Nexium, Diovan, and Valcyte totaled $8 billion last year. Delays to generic Diovan have grossed Swedish drugmaker Novartis $100 million a month. Cash-paying Americans, as usual, are hurt most by these shenanigans as they continue to pay high prices for brand-name medication. Taxpayers should also be unhappy, since they are footing the bill for these brand name drugs purchased through programs like Medicare and Medicaid.
There is, however, a bit of good news on the horizon – the European Medicines Agency, an Agency under the European Union that evaluates medicinal products (like the FDA), will reinstate the good manufacturing practices certificate for Ranbaxy’s Toansa plant, which was slated to produce generic Nexium and Diovan. Hopefully, these improvements are good enough for the FDA.
In the meantime, so you’re not held hostage to drug price insanity, you can find these brand medications internationally at amazing discounts. Feel free to compare their prices:
Diovan – save 70%
Valcyte – save 70%
Nexium – save 93%
Tagged with: Diovan, Nexium, Ranbaxy, Valcyte
by Gabriel Levitt, Vice President, PharmacyChecker.com and Sam Werbalowsky, Pharmacychecker.com | Jun 13, 2014 | Drug Importation, Drug Prices
A few weeks back we wrote about drug affordability problems related to high deductible Obamacare silver plans. A new report finds problems across all four tiers for patients requiring expensive specialty drugs. Many plans have co-insurance rather than a fixed co-pay for these medications, which means patients pay a percentage of a drug’s price rather than a flat fee. In fact, over 50% of bronze, silver, and gold plans studied had co-insurance rather than fixed co-pays for specialty drugs. That compares to only 38% for platinum plans.
According to Wellmark, “Specialty drugs are prescription medications that require special handling, administration or monitoring. These drugs are used to treat complex, chronic and often costly conditions, such as multiple sclerosis, rheumatoid arthritis, hepatitis C, and hemophilia.”
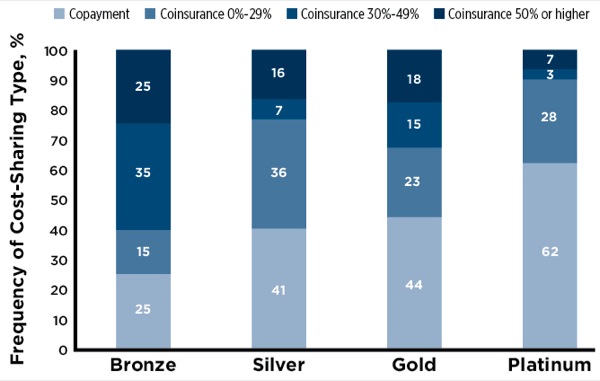
Pamela Morris, of Zitter Health Insights, said “A lot of times, if someone has coinsurance their first exposure to OOP [out of pocket costs] is at the pharmacy, where they may be unsure if they’ve met their deductible or if the costs are purely coinsurance.”
So how big could this price shock be? Let’s look at Tecfidera, a sample oral Multiple Sclerosis drug. The cash price is around $6,000 for 60 capsules of the 120 mg dose. Even if your co-insurance is 25%, that’s $1,500. You can purchase the same amount for $1,200 from an international online pharmacy. Still expensive, but a $300 savings is nothing to scoff at. And it’s likely that the co-pay would be even more than 25% in which case the international savings could be much higher.
Gleevec, a medication used to treat certain types of leukemia, is around $29,000 for 90 pills. That will cost you $7,500 if your co-insurance is only 25%. Using an international online pharmacy, you can purchase 90 pills of generic Gleevec for $725 from a Canadian pharmacy. This may even be cheaper than using Novartis’s patient assistance program for brand name Gleevec. The program has strict eligibility requirements but is worth pursuing if you believe you’re eligible.
We’re sorry to report that many specialty meds may not be safe to order from an international online pharmacy. Some might be extremely temperature sensitive, others are administered in a clinical setting, only sold by specialty pharmacies, and some aren’t even approved for sale outside the U.S. For some specialty drugs, the savings might not even be that great, as prices are high globally!
We promise to research all avenues of savings for these medications and report back to you soon…
Tagged with: Gleevec, Obamacare, specialty drugs, tecfidera
by Gabriel Levitt, Vice President, PharmacyChecker.com and Sam Werbalowsky, Pharmacychecker.com | Jun 6, 2014 | Drug Importation, Drug Prices, Generic drugs
Americans who take Celebrex to fight arthritis may be pleased to know that a generic version has been approved by the FDA. The New York Times’ coverage reports, in a somewhat predictive fashion, that generic drugs “can cost 30 to 80 percent less than the branded products.” While that’s true, don’t expect generic Celebrex to be so cheap. What’s most likely is that its price will initially be around 80%, and then creep downwards.
Millions of Americans have seen this pricing trend over the past few years, as many popular medications have recently gone generic. A patent’s expiry does not necessarily mean cheaper drugs, at least immediately. That’s because the FDA grants marketing exclusivity for a generic to a single drug company for six months, so only two drug companies – the brand name manufacturer and the first generic manufacturer – are competing. As more drug companies enter the market the price will eventually cost a fraction of the brand name counterpart. But that first generic to market will usually only be about 20% cheaper than the brand.
When atorvastatin (generic Lipitor) first came out you could actually save a lot of money by purchasing the brand from an international online pharmacy instead of the generic from a U.S. pharmacy. Now, with many companies manufacturing atorvastatin, generic Lipitor in the U.S. can be found for about $15 per month if you use a discount card. International online pharmacies that once had a leg up on U.S. pharmacies lose big once competition drives U.S. generic drug prices down.
A perfect example is the popular antidepressant Cymbalta. Currently, the cash price of a 90 day supply of duloxetine (generic Cymbalta, 60 mg) is around $250 – and that’s after using a discount card at the pharmacy. A 90 day supply of brand name Cymbalta is only $90 when ordered from an international online pharmacy – a 64% discount!
We expect this pricing pattern for Abilify, Gleevec, Crestor, and many other drugs coming off patent down the pipeline. In the initial phase of a new generic’s release, the brand version from an international online pharmacy will probably be much cheaper, but eventually your neighborhood pharmacy will be your best bet. So if you take Celebrex expect to celebrate a low cost U.S. generic in early 2015!
Tagged with: Atorvastatin, Celebrex, Cymbalta, Patent Cliff





