by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Jan 8, 2015 | Counterfeit Drugs, Drug Prices, Generic drugs, Personal Drug Importation
I wish I was joking about the racy headline above. The United States is not the only great power in which citizens go without medication because of cost. The cancer drug Gleevec (imatinib), made by Novartis, costs 23,500 yuan, or about U.S. $3,783, per month, in China. Gleevec is not covered by health insurance in China so people there must pay for it out of pocket. Ten years ago, Lu Yong was diagnosed with chronic myelocytic leukemia and was prescribed Gleevec. After facing bankruptcy due to his drug costs, Lu discovered a generic version of Gleevec, called Veenat, and began purchasing it by mail-order from India where it is an approved drug, at a cost of only 3,000 yuan, or about $482, per month — 87% less than the brand name drug.
Lu’s condition improved quickly using the generic version. He began to help people with leukemia who he met online obtain Veenat. Now, according to the English edition of Caixin, an independent Beijing-based media outlet, he is facing criminal charges for credit card fraud and selling counterfeit medication. The same story was covered by official Chinese media under the headline “Leukemia patient prosecuted for buying pills overseas.” Lu has helped 1,000 people with leukemia obtain treatment. Three hundred of them are petitioning the authorities to have his name cleared.
The medications involved are real and clearly life-saving! So why is Lu being prosecuted for counterfeit drugs? Under Chinese law, any drug not specifically licensed for sale in China, even a genuine medication lawfully manufactured by an authorized drug company, is considered counterfeit.
The charge of credit card fraud was based on Lu’s using a foreign credit card to make the purchases. Lu said he did so because using domestic Chinese bank-issued credit cards for international purchases is nearly impossible.
Lu was not charged for procuring his own cancer medication. The charges were for directly facilitating the purchase of the drug for 1000 people, who consequently regard Lu as a hero.
When people can’t afford to obtain life-saving medication locally, the U.S, and all countries, should consider themselves morally obligated to expressly permit their citizens to obtain it internationally.
Tagged with: China, Gleevec, imatinib
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Nov 21, 2014 | Drug Importation, Drug Prices, Generic drugs, Personal Drug Importation, Politics
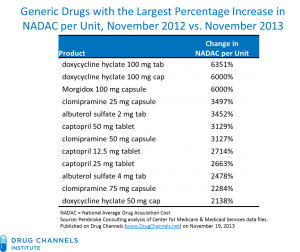
From Pembroke Consulting, published on DrugChannels.net
Yesterday, Senator Bernie Sanders (I-VT) held a hearing in front of the Senate Health, Education, Labor and Pensions Subcommittee on Primary Aging and Health entitled “Why Are Some Generic Drugs Skyrocketing in Price?” Bizarre drug price increases of 1,000% and up are becoming more common. This problem is not new: in the beginning of this year the People’s Pharmacy reported a 6,000% increase in the price Doxycycline!
Senator Sanders noted that the price of Digoxin, which treats congestive heart failure, has increased 883% from 12 cents to $1.06 per pill from 2013 to 2014. Migraine drug Divalproex Sodium ER spiked 881% from 27 cents to $2.38 per pill.
We are always ecstatic when Congress scrutinizes the obscene drug prices in America, and we applaud Sen. Sanders, but our focus is on what can be done now so that people today don’t go without needed medications. In his introductory remarks, Sen. Sanders said: “Drug prices in the country are by far the highest in the world.” Let’s elaborate on that. (more…)
Tagged with: Bernie Sanders, Digoxin, Divalproex Sodium ER, FDA, generic drugs, Pembroke Consulting
by Gabriel Levitt, Vice President, PharmacyChecker.com and Sam Werbalowsky, Pharmacychecker.com | Sep 5, 2014 | Drug Prices, Generic drugs, Specialty Drugs
Specialty drugs have been in the news for their exorbitant prices lately. Gilead Sciences’ Hepatitis C cure Sovaldi has received media exposure for costing $84,000 and in 2012, when Memorial Sloan-Kettering Cancer Center refused to use the colon cancer medication Zaltrap because of its $11,000 a month price, the manufacturer responded by offering discounts of 50%. Will these high prices come way down once the medications go generic?
A new study from the National Bureau of Economic Research, examined costs and utilization of specialty drugs (specifically cancer meds) as generic versions are introduced. Generally, prices for generic drugs drop as more manufacturers produce them due to price competition. This should presumably happen for specialty drugs, but there’s a catch. Many specialty drugs have a small user base and some of them are formulated as solutions or injection, which may require more specialized and expensive manufacturing processes compared to traditional oral drugs (i.e pills, liquids). For those reason, the drugs price discrepancy between brands and generics is not as great among specialty medications compared to regular medications.
The study didn’t analyze the best way to actually pay for these medications. But a recent analysis by HealthPocket took a look at Obamacare plans and specialty meds. Check that out here. We are still researching paying for specialty drugs and will have our own analysis and tips for saving at some point in the future.
Keep in mind that over time specialty drugs, such as Sovaldi, will go generic in the U.S. and be prices considerably lower than the brand. But unlike many pills for high blood pressure, depression, or cholesterol, you won’t find $4 Zaltrap at your local Rite-Aid anytime soon.
I wish I had a more concrete answer and analysis on the prices and access to specialty meds. It’s something that we here at PharmacyChecker.com are keeping an eye on, and we will certainly have updates in the future.
Tagged with: Gilead Sciences, National Bureau of Economic Research, Obamacare, Sloan-Kettering, Sovaldi, specialty drugs, Zaltrap
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Aug 15, 2014 | Drug Prices, Drug Safety, FDA, Generic drugs, New Drugs
Most people think newer is better, but according to a study published in Health Affairs that might not be the case for prescription drugs. In short, the new study shows that drugs approved by the U.S. Food and Drug Administration after enactment of the Prescription Drug User Fee Act of 1992 (PDUFA), a bill that led to more expeditious drug approvals funded by drug companies, were more likely to have safety problems than ones approved before PDUFA. These findings are not only relevant to drug safety, but also to drug savings. Older drugs are often sold as generics and, thus, will have much lower co-payments than new drugs. For those paying out-of-pocket, the cost of a generic is often 80% less than the brand.
The study analyzed 748 drug approvals between 1975 and 2009. The approvals were of new molecular entities not for generic versions of existing brand-name drugs. Before PDUFA the chances that safety issues would arise involving approved new drugs was 21.2%; after PDUFA it increased to 26.7%.
According to the lead author, “The FDA needs to make sure drugs are safe before they’re approved, not rush to judgment in order to meet artificial deadlines.” Not surprisingly, FDA and the Pharmaceutical Researchers and Manufacturers of America, take issue with the study. Their main points are that PDUFA helped speed up important drug approvals and get medications to patients faster and it improved the predictability of FDA’s system of drug approvals.
Regulations for marketing and manufacturing new drugs can save people and they can kill people. If the regulations are too rigid then patients won’t get needed medications fast enough. Or regulations can increase manufacturing costs resulting in unaffordable drug prices. If regulations are too weak then drugs will be less safe and effective. While in my opinion the study clearly has merit, PDUFA is helpful. Before its passage, drug approvals were lagging far behind other advanced economies in Europe.
Furthermore, the study does not show “causality,” meaning it does not prove that faster drug approvals after PDUFA led to less safer drugs. Nonetheless, it’s understandable that a drug with a long history of safe and effective use, accompanied by few side effects, is more trustworthy than a newly approved drug since the long term effects of the latter are unknown.
But what does this all mean for consumers and drug savings? (more…)
Tagged with: Atorvastatin, Brintellix, Crestor, Prozac, SSRI, Statin
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Jun 25, 2014 | Drug Prices, Drug Safety, Generic drugs

Toprol XL, a prescription drug that treats high blood pressure and is shown to lower the risk of heart attacks, is in the news due to recalls of some generic versions and because some doctors are finding the generics doesn’t always work as well as the brand. Consumers taking a generic version (metoprolol succinate extended-release), might want to switch back to the brand, but that could raise their drug bills substantially
The issue has also re-ignited the topic of problems with Indian drug quality. Wockhardt and Dr. Reddy’s Laboratories, both based in India, have pulled 100,000 bottles of their respective metoprolol succinate extended-release products because their pills were not dissolving properly. For extended release drugs, this can be a big problem with a drug’s efficacy.
So where can you buy an American made version of this drug? I believe that would be very hard to do. Brand name Toprol XL sold on U.S. pharmacy shelves is a product of Swedish company AstraZeneca and, according to the drug’s labeling, made in India. There are American companies that make metoprolol succinate as well, such as Mylan, but they manufacture the drug in India, too. Some Toprol XL generics were made in America, but that didn’t work out so well due to manufacturing problems here at home.
Dollars and Sense – You can save 70% on the brand!
In the U.S., generic metoprolol succinate costs about 45 cents a pill. If you want brand name Toprol XL (100mg), the cost is about $2 apill. However, if you only want the brand name product and want to spend a lot less, it can be purchased from a verified international online pharmacy for as little as 60 cents a pill, just a bit more than the cost of a generic in the U.S., and a 70% savings on the U.S. brand price.
Compare Toprol XL drug prices on all strengths on PharmacyChecker.com.
Tagged with: AstraZeneca, Dr. Reddy's Labratories, Toprol XL, Wockhardt
by Gabriel Levitt, Vice President, PharmacyChecker.com and Sam Werbalowsky, Pharmacychecker.com | Jun 20, 2014 | Generic drugs, Pharmaceutical Industry
Americans are used to finding low-cost generic medications about six months to a year after they’re approved by the FDA. Think Lipitor, Plavix and Lexapro, which are all priced about 80% lower as generics.
Unfortunately, patients who had been looking forward to newly-approved generic versions of Nexium, Diovan, or Valcyte will have to keep waiting as a knot of legal and regulatory guidelines delay their U.S. release. The FDA approved Indian drugmaker Ranbaxy’s generic versions of these meds but has banned the plants to be used for their production from exporting products to the U.S., due to findings of substandard manufacturing practices.
You’d think that another drug manufacturer could just make and market the drug, but the U.S. approval system doesn’t allow that. The FDA grants six months of exclusive marketing rights to the company that first gains approval for a generic drug. We wrote about this process two weeks ago in our blog post covering generic Celebrex. Even though Ranbaxy’s plants can’t export generic versions of Nexium, Diovan, and Valcyte to the U.S., it still retains marketing exclusivity in the U.S. market!
This is good news for brand name drug makers and bad news for consumers. Global sales for Nexium, Diovan, and Valcyte totaled $8 billion last year. Delays to generic Diovan have grossed Swedish drugmaker Novartis $100 million a month. Cash-paying Americans, as usual, are hurt most by these shenanigans as they continue to pay high prices for brand-name medication. Taxpayers should also be unhappy, since they are footing the bill for these brand name drugs purchased through programs like Medicare and Medicaid.
There is, however, a bit of good news on the horizon – the European Medicines Agency, an Agency under the European Union that evaluates medicinal products (like the FDA), will reinstate the good manufacturing practices certificate for Ranbaxy’s Toansa plant, which was slated to produce generic Nexium and Diovan. Hopefully, these improvements are good enough for the FDA.
In the meantime, so you’re not held hostage to drug price insanity, you can find these brand medications internationally at amazing discounts. Feel free to compare their prices:
Diovan – save 70%
Valcyte – save 70%
Nexium – save 93%
Tagged with: Diovan, Nexium, Ranbaxy, Valcyte




