by Tod Cooperman, MD, President, PharmacyChecker.com, and Gabriel Levitt, Vice President, PharmacyChecker.com | Feb 26, 2015 | Drug Importation, Government, Personal Drug Importation
Unfortunately, a Maine state law that was created to help people access lower cost medication from licensed pharmacies in Australia, Canada, New Zealand and the United Kingdom, was invalidated yesterday in a decision by federal court Judge Nancy Torresen. Basically the judge, invoking a legal doctrine called “preemption,” concluded that federal law beats state law when it comes to foreign commerce, and since federal law technically bans personal drug importation under most circumstances, Maine’s law is trumped. I’ll return at the end to deal with a little legalese fun (but not too much!).
Taking a walk down memory lane here: personal drug importation programs in Maine, such as one operated for the City of Portland, Portland Meds since 2004, which has helped Americans save many millions of dollars, were shut down in 2012 by former State Attorney General William Schneider. The programs were shut down because Maine’s pharmacy groups persuaded AG Schneider that Canadian and all foreign pharmacies should be stopped from mail order pharmacy sales into Maine because they are not licensed in Maine. Most U.S. states require pharmacies based elsewhere to obtain an out-of-state pharmacy license if they want to sell medication by mail to their residents. While there are exceptions, most states do not allow pharmacies in other countries to obtain an out-of-state license.
Maine legislators were angered by this action and passed a law, LD 171 “An Act To Facilitate the Personal Importation of Prescription Drugs from International Mail Order Prescription Pharmacies,” that exempted licensed pharmacies in Australia, Canada, New Zealand, and the UK from having to obtain an out-of-state Maine pharmacy license. Not only was this law passed on a bi-partisan basis but the vote was overwhelming: Maine’s House voted 107-37: the Senate voted 30-4. And with that the personal drug importation programs resumed.
The law was invalidated, now what?
Programs like Portland Meds will not necessarily shutdown. We’ll have to wait and see what happens. But if they do shutdown then thousands of Mainers will be paying more for their medications. More seriously, some Mainers will likely end up skipping their medications because the prices at their local pharmacies are too high for them. Back in 2012, an owner of one company that worked with CanaRx, a Canadian pharmacy benefit company, admitted that by working with licensed foreign pharmacies his company saved money: but there was more to the story than simply a company saving money. Quoting a journalist from the Bangor Daily News:
While acknowledging that Hardwood Products “as a company is trying to save money,” Young said his greatest fear is that a spike in costs will spur his employees to stop taking medications for conditions such as diabetes and asthma.
“We have many people here who are hourly employees,” he said. “We pay a fair wage, but the impact out of the family net income will be significant. More important than the money is the health and well being of the employees and their families. What dollar figure do you put on that?”
…but all hope is not even close to lost! Americans still have access to safe and more affordable medication available online, and, again, Maine’s programs have not yet shut down. Equally as important to the longer term cause of prescription justice, the ruling leaves the door open for the State of Maine to appeal the decision up the legal food chain to the 1st Circuit Court of Appeals in Boston. If Maine wins then other states may follow its lead by passing similar legislation to promote access to lower costs medications from other countries.
I’m pretty certain that, with the requisite political will from Maine’s legislators, citizen rabblerousing, and some good legal marksmanship, there are ways to overcome and defeat Judge Torresen’s ruling.
To conclude, I’d like to challenge something Judge Torresen opined in her ruling to nullify Maine’s foreign pharmacy law:
“Congress enacted the FDCA [Food, Drug and Cosmetics Act] to bolster consumer protection against harmful products.”…In furtherance of this purpose, Congress has created a complex regulatory scheme covering the importation of pharmaceuticals into the United States…
Is that so? Maybe…in part. However, I believe that banning Americans from importing lower cost and safe prescription medication from licensed pharmacies for their own use does nothing to bolster consumer protection against harmful products but quite a lot to bolster protection of big drug company and U.S. chain pharmacy profits. I know that the ban impedes Americans from taking medications they need and forces more financial hardship. Are these facts that could hold up in court? I think so.
Tagged with: CanaRx, legal, Maine, Torresen
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Dec 30, 2014 | Advocacy, Drug Importation, Personal Drug Importation, Politics
The fight for access to safe and affordable medication will continue in 2015. As 2014 ends we find the price of medication continuing to escalate. AARP’s recent drug price report showed that brand name drug prices had increased by 13% in 2013, eight times the rate of inflation. The costs of generic drugs are going through the roof, some by astronomical amounts – to the tune of thousands of percent. Then of course 2014 brought us Sovaldi at $1000/pill, which is just the tip of the iceberg, as many more outrageously priced “specialty medications” are coming down the pipeline in 2015. New drugs that save lives and help people get better are great…but only if they are affordable.
In the holiday spirit, Senator Amy Klobuchar from Minnesota published a Christmas-themed op-ed last week called “How the Drug Companies Play Scrooge,” as in Ebenezer Scrooge, the greedy miser in Charles Dickens’ famous story A Christmas Carol. Klobuchar’s ghosts of Christmas past, present and future are, respectively, rampant drug price increases; the highest drug prices in the world by far; and the continuing assault on the pocketbooks of Americans by pharmaceutical companies unless Congress acts. Interestingly, Sen. Klobuchar’s metaphor compares Scrooge to Congress (not the pharmaceutical industry). She writes: “if Ebenezer Scrooge can be transformed from a crotchety, thoughtless, “bah humbug” miser to a generous steward of good will to all after only one night of ghostly visits, certainly there is hope for Congress.”
What can Congress do to end its Scrooge-like protection of big pharma? Sen. Klobuchar recommends three legislative solutions. Congress should pass legislation that would allow Medicare to negotiate drug prices with pharmaceutical companies under the same protocols permitted to the Department of Veterans Affairs. Currently, federal law actually bans such negotiations. Under one estimate, due to the VA’s ability to negotiate prices, drug prices are 40% lower when obtained through the VA than through Medicare.
Ending what Sen. Klobuchar calls “illegal pay-to-delay” deals between brand name and generic pharmaceutical companies, which postpone market availability of lower cost generic drugs, is another solution that can be addressed legislatively. Legislation introduced by Klobuchar and Sen. Charles Grassley would give the Federal Trade Commission more authority to stop those deals. Sen. Klobuchar asserts that the savings from ending pay-to-delay could be $4.7 billion for the U.S. budget and $3.5 billion for consumers.
Last but not least, Sen. Klobuchar recommends helping American consumers to personally import lower cost medication by passing The Safe and Affordable Drugs from Canada Act. This legislation, co-sponsored by Sen. John McCain from Arizona, would essentially codify the current practice of Americans buying lower cost medication from Canadian pharmacies. We support this bill but believe it needs to be expanded to include pharmacies in many other countries from which lower cost and safe medication can be and currently are obtained.
Congressional opponents of Klobuchar’s personal drug importation bill, the most vociferous among them surely raking in huge donations from big pharmaceutical companies, will argue that the Act will open the door to counterfeit drugs and rogue online pharmacies. The fact that counterfeit drugs and rogue online pharmacies exist, however, is not an argument against facilitating safe personal drug importation from verified international online pharmacies. Five million Americans already import medication for their own use. Consumers who purchase from safe international online pharmacies, such as those in the PharmacyChecker.com Verification Program, are able to save thousands of dollars a year. For many, personal importation of lower cost medication is the only option for obtaining needed prescription drugs.
While the FDA doesn’t prosecute Americans who import medications for their own use, federal law still holds that, under most circumstances, it is a crime!! It’s understandable that prescription drug importation meant for re-sale in U.S. pharmacies is regulated to that extent that those who violate the laws are subject to penalties. In contrast, people who need to import medication for their own use because they can’t afford the prices at U.S. pharmacies should not be subject to criminal enforcement of any kind, ever. So I hope that Senator Klobuchar and her colleagues, in addition to her recommendations identified above, introduce and pass a bill to amend federal law to decriminalize personal drug importation. By doing so Congress would bring prescription justice to Americans who are haunted by the scary ghosts, past, present and future, of the pharmaceutical industry.
Happy Holidays and New Year from PharmacyChecker.com!!
Tagged with: decriminalization, klobuchar, legislation
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Nov 21, 2014 | Drug Importation, Drug Prices, Generic drugs, Personal Drug Importation, Politics
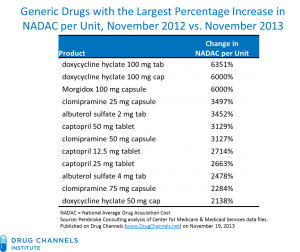
From Pembroke Consulting, published on DrugChannels.net
Yesterday, Senator Bernie Sanders (I-VT) held a hearing in front of the Senate Health, Education, Labor and Pensions Subcommittee on Primary Aging and Health entitled “Why Are Some Generic Drugs Skyrocketing in Price?” Bizarre drug price increases of 1,000% and up are becoming more common. This problem is not new: in the beginning of this year the People’s Pharmacy reported a 6,000% increase in the price Doxycycline!
Senator Sanders noted that the price of Digoxin, which treats congestive heart failure, has increased 883% from 12 cents to $1.06 per pill from 2013 to 2014. Migraine drug Divalproex Sodium ER spiked 881% from 27 cents to $2.38 per pill.
We are always ecstatic when Congress scrutinizes the obscene drug prices in America, and we applaud Sen. Sanders, but our focus is on what can be done now so that people today don’t go without needed medications. In his introductory remarks, Sen. Sanders said: “Drug prices in the country are by far the highest in the world.” Let’s elaborate on that. (more…)
Tagged with: Bernie Sanders, Digoxin, Divalproex Sodium ER, FDA, generic drugs, Pembroke Consulting
by Gabriel Levitt, Vice President, PharmacyChecker.com and Sam Werbalowsky, Pharmacychecker.com | Sep 17, 2014 | Advocacy, Drug Importation, Personal Drug Importation
The opinions and research of two Americans, published this week in local newspapers, epitomize the position of millions of Americans: drug prices are too high and safe personal drug importation is a smart way to afford medication. Tom Kennedy compared prices between the U.S. and Canada in 2003, and he is doing it again 11 years later. His guest opinion in the Billings Gazette has shown that U.S. prices have increased 153% for the drugs he tracked since 2003, far outpacing the rise in income and cost of living. He has some good economic insight and analysis, and I recommend reading his whole opinion, which you can find here.
David Di Saia, from North Providence, Rhode Island, found that he could save $480 a year by using a Canadian pharmacy instead of the pharmacy associated with his Medicare plan. And that’s just the savings for one medication! Imagine the savings if he had to order more than one drug. You can read his story, which is “sad, but true” on the Valley Breeze website.
Tagged with: Billings Gazette, Drug Prices, Valley Breeze
by Gabriel Levitt, President, PharmacyChecker.com and Prescription Justice | Jul 10, 2014 | Drug Importation, Online Pharmacies, Politics
In our recent public comments to the U.S. Food and Drug administration, we invoked your concerns with new FDA regulations to implement Section 708 of the Food and Drug Administration Safety and Innovation Act (FDASIA) for destroying safe (and affordable) medications imported for personal use. We issued a press release on the issue as well. Here are three of almost 2000 comments:
Morton Ross, Palm Harbor, FL 2014-04-03, “The Meds I take daily, are the difference between ‘Life and Death’. I cannot afford the higher prices at local pharmacies.”
Darilyn Schlie, Fort Worth, TX 2014-04-03, “Without the ability to go outside the US, I will not be able to afford the medication I need.”
James Marshall, Nashville, TN 2014-04-03, “I have emphysema and could not afford my medications if not for being able to order some of them from outside the USA.”
We need more voices! Working with RxRights.org, you can send a message to the Secretary of Health and Human Services stating your concerns, asking that she prevent morally unjust and dangerous refusals and destructions of imported drugs for personal use. The messaging process is easy and together we can win this!
The situation is not dire…yet. The regulation has not taken effect and it’s uncertain what its impact will actually be. Currently, the conventional wisdom holds that the chances of your prescription order being detained by U.S. Customs is less than 1%. What we don’t know is if the new regulations will change that dramatically.
What we do know is that FDA issued a proposed rule in May to implement Section 708, which is deceptively called “Destruction of Adulterated, Misbranded, or Counterfeit Drugs Offered for Import.” Destroying adulterated and counterfeit drugs sounds like a good plan but many, if not most, ‘misbranded drugs’ sold in foreign pharmacies are actually the same drugs you can buy at your local pharmacy just with different labeling and packaging. Though sometimes manufactured in a plant not registered with the FDA, they are manufactured in plants registered with another drug regulatory authority. Or they might be a generic version of a brand name drug that is approved in the U.S. but not yet off-patent here. These examples of real, safe and effective medications are usually deemed ‘misbranded’ or ‘unapproved’ by FDA. For more on this see my New York Times op-ed.
To Congress’ credit, before Section 708 goes into effect, regulations must be drafted requiring that consumers, 1) receive notification that their prescription drug orders have been refused import and 2) are provided “appropriate due process” to defend their drug imports before they are destroyed. Unfortunately, as I read it, FDA’s proposed rule did not assure consumers “appropriate” due process. In our comments we proposed that FDA clearly explain to consumers why their drug imports were refused and exactly how consumers can provide testimony to prevent the FDA from destroying their imported drugs. In the final rule, consumers should be able to successfully defend refused drug imports of safe and prescribed medication to have them released not destroyed. They need those medications to safeguard their health, and sometimes even lives.
We also proposed a revision to FDA’s personal drug importation policy so that safe personally imported medication from certain countries with very strong pharmaceutical regulations and pharmacy standards would not be detained or refused. We also recommended continuing actions to shutdown dangerous rouge pharmacy sites but not obtusely conflating them with safe international online pharmacies.
See below to learn more and advocate.
Petition the Government!
Comments by Americans concerned with Section 708
Comments by PharmacyChecker.com to the FDA on Section 708
by Gabriel Levitt, Vice President, PharmacyChecker.com and Sam Werbalowsky, Pharmacychecker.com | Jun 13, 2014 | Drug Importation, Drug Prices
A few weeks back we wrote about drug affordability problems related to high deductible Obamacare silver plans. A new report finds problems across all four tiers for patients requiring expensive specialty drugs. Many plans have co-insurance rather than a fixed co-pay for these medications, which means patients pay a percentage of a drug’s price rather than a flat fee. In fact, over 50% of bronze, silver, and gold plans studied had co-insurance rather than fixed co-pays for specialty drugs. That compares to only 38% for platinum plans.
According to Wellmark, “Specialty drugs are prescription medications that require special handling, administration or monitoring. These drugs are used to treat complex, chronic and often costly conditions, such as multiple sclerosis, rheumatoid arthritis, hepatitis C, and hemophilia.”
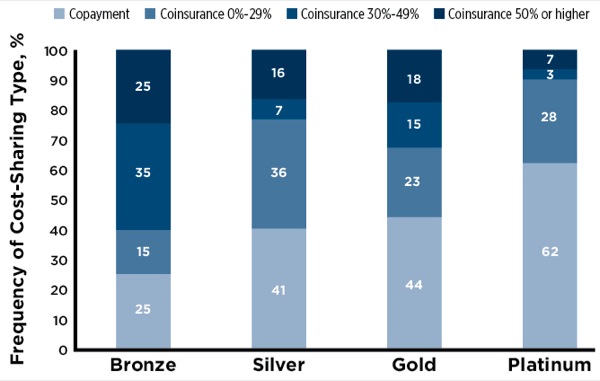
Pamela Morris, of Zitter Health Insights, said “A lot of times, if someone has coinsurance their first exposure to OOP [out of pocket costs] is at the pharmacy, where they may be unsure if they’ve met their deductible or if the costs are purely coinsurance.”
So how big could this price shock be? Let’s look at Tecfidera, a sample oral Multiple Sclerosis drug. The cash price is around $6,000 for 60 capsules of the 120 mg dose. Even if your co-insurance is 25%, that’s $1,500. You can purchase the same amount for $1,200 from an international online pharmacy. Still expensive, but a $300 savings is nothing to scoff at. And it’s likely that the co-pay would be even more than 25% in which case the international savings could be much higher.
Gleevec, a medication used to treat certain types of leukemia, is around $29,000 for 90 pills. That will cost you $7,500 if your co-insurance is only 25%. Using an international online pharmacy, you can purchase 90 pills of generic Gleevec for $725 from a Canadian pharmacy. This may even be cheaper than using Novartis’s patient assistance program for brand name Gleevec. The program has strict eligibility requirements but is worth pursuing if you believe you’re eligible.
We’re sorry to report that many specialty meds may not be safe to order from an international online pharmacy. Some might be extremely temperature sensitive, others are administered in a clinical setting, only sold by specialty pharmacies, and some aren’t even approved for sale outside the U.S. For some specialty drugs, the savings might not even be that great, as prices are high globally!
We promise to research all avenues of savings for these medications and report back to you soon…
Tagged with: Gleevec, Obamacare, specialty drugs, tecfidera




